Developmental bias: An interview with Wallace Arthur
by Wallace Arthur & Katrina J Falkenberg
10 July 2018

A Geophilus centipede. Image: Fritz Geller-Grimm, CC-BY-SA-2.5.
Wallace Arthur is an Emeritus Professor of Zoology at the National University of Ireland in Galway. He is a renowned evolutionary developmental biologist, working across disciplinary boundaries to understand the evolution of animal body plans and segmentation, a subject on which he has published numerous scientific papers and books, as well as popular science pieces. Wallace is one of the leading advocates of an important role for developmental bias in evolution, a view that he brought to prominence through his academic monograph Biased Embryos and Evolution and textbook Evolution: A Developmental Approach. Katrina Falkenberg is the science communication and outreach officer for the EES research program. She interviewed Wallace about developmental bias and its role in evolution.
Katrina: Thank you for talking with us today Wallace. Can you begin by describing what developmental bias is, and whether (and how) it differs from developmental constraint?
Wallace: Developmental bias describes the notion that development plays an active role in evolution by making certain phenotypic variants more likely than others. This is something that has fascinated me for many years. I didn’t like the much older term, developmental constraint, however, because it sounded negative. I read a paper of Steve Gould’s1 which said that we can use this term in both a positive and a negative way because in times gone by, people said things like “I feel constrained to speak!” meaning as we would say, “I feel compelled to speak!”. Gould claimed that it’s always had this double meaning so it’s fine to use it. But I never believed that argument because I think that a usage that has disappeared maybe a couple of centuries ago is probably not going to come back.
I’m one of the large group of people who think that development is important in evolution, both in an active way as well as being a target for natural selection. So I really wasn’t satisfied with having a negative word or phrase, developmental constraint, as the kind of cover term for this whole notion, which is how some people were using it. In 2001,2 I said wait a minute, wouldn’t it be nice if we could have a phrase for development influencing evolution in an active sort of way, and that could be a cover term. Then you could have within that the negative, developmental constraint, and the positive, and in this paper I invented the term, developmental drive. Developmental bias already existed but was used by fewer people than developmental constraint. So at the time, my view was that developmental bias is the cover term, and takes two forms, developmental constraint and developmental drive.
By focusing on constraint, people who are interested in furthering our understanding of how development is involved in the evolutionary process immediately put themselves at a disadvantage, compared with, for example, the conventional population genetics school of thought, which has natural selection. Natural selection sounds quite positive, so if you are hooked to something called developmental constraint you’re getting engaged in an interaction where you put yourself at a disadvantage at the beginning. This reminds me of Thomas Henry Huxley’s invention of the word agnosticism. He always felt that he was at a disadvantage arguing against theists or theologists, or indeed atheists, because there was no name for his stance. He was a dyed-in-the-wool agnostic, but the term didn’t exist until he invented it and he had previously felt that he was on weak ground. So, I think that if you’re involved in a debate, it’s better first of all to have a name for your stance, and secondly to have one that doesn’t have negative connotations.
Katrina: What are some of your favorite examples of developmental bias?
Wallace: For quite a few years I’ve been studying the example of evolution of segment number in the arthropod group we call the centipedes. There are approximately 3000-4000 species of centipedes, with the biggest group of these being the geophilomorphs, the soil-dwelling centipedes. If you count the number of pairs of legs, the lowest in any species is 27 pairs and the highest is 191 – hugely variable – and if you plot the distribution of segment number against number of species that have that segment number, you get a curve. However, the curve is chopped into thin columns because all the even numbers don’t exist. You can think of that as developmental constraint in a negative sense that somehow the even numbers are prohibited by the developmental process or in a positive sense that there is developmental drive into the odd numbers. Or it could be that natural selection somehow acts against even numbers of leg pairs, though this doesn’t really make much sense.
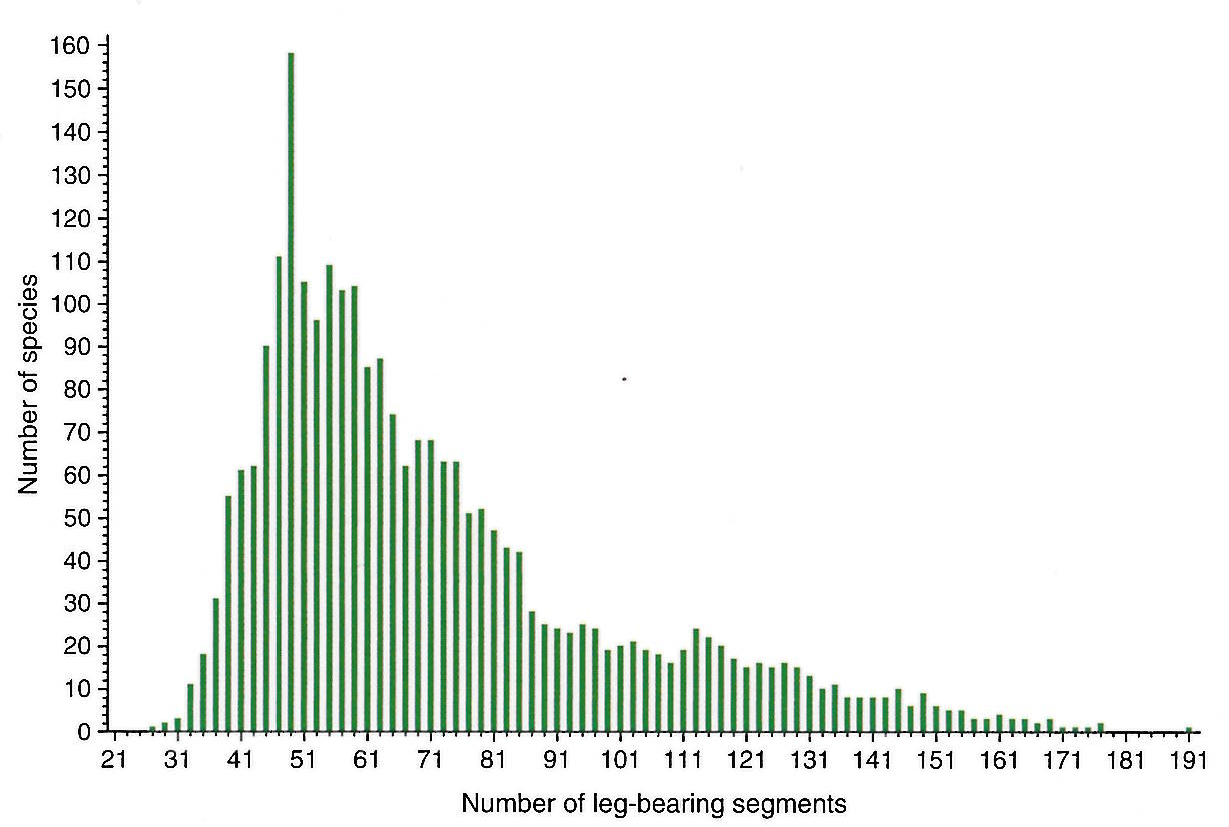
Distribution of leg-bearing segment numbers in the Geophilomorph centipedes. Image: Figure 13.11 from Evolution: A Developmental Approach, redrawn from 3.
The final twist to the story is that from patterns of gene expression it’s quite clear that the venom claws that exist in every species of centipede on the segment immediately behind the head are really modified walking legs. If you count them, then the number of pairs is always even rather than always odd. That coincides with, for example, a model of development which says you make pre-segmental units which turn out to be pairs of segments and so you can’t get an uneven number of segments. Whether you see that as constraint against something (one type of number), or a drive into the other type of number, it’s the same.
Another example of bias came out of work by Pere Alberch and Emily Gale, where they showed certain patterns of digit reduction in amphibians.4,5 Take two groups of amphibians, (i) the anura, the tailless ones, the frogs and toads, and (ii) the urodeles, the ones with tails, the newts and salamanders. What you find is that when digit reduction occurs in particular lineages of frogs on the one hand and newts on the other, the digits that are preferentially lost are consistent within a group but different between the groups. There are a lot of cases of digit loss, so you have a lot of quasi replicates within the frog group and within the newt group. Alberch, Gale and others then did some experimental work trying to modify frog and newt limb development. What they found was that when you tried to modify, using various techniques, limb development in order to reduce digits, the ones that you lost most readily in the frog group through experimental procedures were the same ones that tended to get lost evolutionarily. In the newt group where the evolutionary pattern was different, nevertheless, when you did experiments on newt development you found that the losses again corresponded with the evolutionary losses. And that to me definitely says that the mechanics of development are involved in the trends that happen in evolutionary lineages.
Another example is the well-known work from Paul Brakefield’s group on eyespot patterns in the butterfly Bicyclus anynana.6,7 What I particularly like about it is that it shows how natural selection and developmental bias interact with each other. They found that when you tried to use artificial selection to override developmental bias in the relative sizes of the eyespot patterns on the wings, selection was able to override the developmental bias.6 But in the second set of experiments, where they tried to get artificial selection to override bias in the color composition of the eyespots, selection was unable to do so.7 That contrasting pair of outcomes I find absolutely fascinating.
Katrina: In your centipede example, you could view developmental constraint and developmental drive as two sides of the same coin. Do you think this is always the case or do you think developmental drive can operate in a different dimension? I’m thinking of the mammalian molar work from Jukka Jernvall’s group,8 for example. By studying the development of molar teeth in mice, they are able to make predictions about the relative size and number of teeth in other rodent species. This gives the impression that the developmental process is not just closing down some options, but actually marking out potential trajectories through morphospace. That seems to be slightly different from a notion of constraint.
Wallace: I originally thought of developmental drive as the opposite of constraint – the positive and negative versions of developmental bias. Now I don’t think it’s that straightforward. One option is to get away from any of these terms and actually become more process orientated. Maybe when we’ve done enough studies of what actually happens during development we’ll come up with better terminology. Of course, maybe we won’t, but I would like to think that that might happen. In the meantime, it’s important to stress that a binary distinction between ‘all possibilities’ (i.e. no constraints, selection has a free reign) and ‘what is not possible’ (because of some ‘absolute’ constraint operating) is much too simple a view. Quantitative bias within the realm of what is possible can significantly shape how and why evolution unfolds in the way that it does. Developmental systems can alter the proportion of variation that occurs on one phenotypic dimension relative to another.

African clawed frog, Xenopus laevis. Image: H Krisp, CC-BY-3.0.
Katrina: Now that we’ve discussed terminology and specific case studies, let’s talk about the general importance of developmental bias in evolution. This is a quote from your own book, Biased Embryos and Evolution (2004, p.192-193):9
“Is the whole new evo-devo endeavour a footnote? I think not. Is the proposal that developmental bias plays a major role in determining the direction of evolutionary change a footnote? Hardly. We are entering an exciting new era of evolutionary theory in which development is at last beginning to make the contribution that it must. To write this new era off as a footnote (and it had already begun when Dawkins wrote those words) is in my view a serious mistake. I would agree that Darwin and Wallace largely solved the external side of the evolutionary puzzle, but the internal side is only now yielding some of its many secrets. And note that it is a ‘side’, not a ‘footnote’; or, if you prefer, an equal partner rather than a bit-part player.”
Could you elaborate on your description of developmental bias as an “equal partner” to natural selection? And as it’s now it’s over a decade since the publication of the book, do you still agree with what you wrote some 14 years ago?
Wallace: I’m pleased to say I do still agree with myself! I think the way forward is to do more studies of a variety of examples and systems to try to somehow solve the kind of deadlock we’re in. To some extent there’s a social dimension to science – one group tends to have its own conferences and another group tends to have other conferences, and never the twain shall meet – and that’s an unhealthy and unproductive situation I think.
However, I suppose the other thing we haven’t yet covered is the notion of internal selection. In one of my other books,10 I got quite excited about this thing called internal selection after reading the book by Lancelot Law Whyte.11 He said that it’s all very well having these examples like Darwin’s finches, where it’s all to do with selection coming from the environment, but what about selection for co-adaptation; what about selection for integration of parts in the organism? Rather than just taking one part like a beak and looking at the relationship between that part and the environment, let’s look at selection operating on the degree to which the organism is an integrated whole.
Whyte was blasted out of the water by many selectionists of a more conventional disposition and I think it’s because people misinterpreted what he was saying. They thought that he was proposing that some kind of selective process was going on within the organism which, at least to me, is nonsensical. But he was actually saying that selection was going on in the normal way that we think of selection as going on, but for internal reasons. That kind of interpretation of internal selection comes very close to some people’s definition of developmental constraint. However, the relationship between internal selection and developmental constraint – are they the same? are they different? – is a hugely difficult area.
Katrina: Internal selection could be viewed as a source of constraint, i.e. a historical explanation for the current existence of constraint or bias, rather than the mechanism by which it operates in the present. Do you agree?
Wallace: That is one possibility, yes. If there is selection early in embryogenesis against some variant because it decreases the integration of the organism as it tries to continue through development, then to me that’s internal selection. You could interpret the result of that internal selection, in terms of the forms that you do and don’t see later in development, as developmental constraint. In this case, exactly what you say would be true. However, I think there’s another kind of constraint that may exist, where a particular developmental system, say a particular species for example, is simply unable to produce certain variants in the first place. In that case, the constraint is not caused by internal selection, it’s caused by something else, something to do with the dynamics of development itself. The centipede segment number example is a case in point.
Katrina: Let’s talk about opposition to developmental bias. Here is a quote from Stephen Jay Gould’s The Structure of Evolutionary Theory (2002, p.1028):12
“orthodox Darwinians have not balked at the negative constructions of constraint as limits and impediments to the power of natural selection in certain definable situations. But they have been far less willing to embrace positive meanings of constraint as promoters, suppliers, and causes of evolutionary direction and change.”
Do you have any thoughts on why this is?
Wallace: I’ve always wondered about this. My academic upbringing as a postgrad was most definitely in the tradition of neo-Darwinian population genetics. I wasn’t yet thinking about the role of development, and although I wasn’t discouraged from thinking about development, my training didn’t give me any leads into that area. Some people do have a tendency to be a bit blinkered and not want to take on board ideas that are coming from let’s say, evo devo, or other approaches. However, population geneticists who feel like that – who feel ‘against’ the developmental approach – may well be in the minority. It would be an interesting sociological experiment to survey population geneticists to see what proportions are of the two leanings.
I think there is concern among those who are ‘against’ the developmental approach, that other approaches to evolution, and perhaps in particular the evo devo one, are somehow threatening to the continued existence of a core population level evolutionary theory based on Darwinian selection. Whether they’re right to worry about that is another interesting question. If you then look at the heterogeneity within the group of people who would claim to be either evo devo or something of that sort, you find quite different views. Some of those people say yes, development is really important but it interacts with natural selection, which is basically what I tend to say. You get other people who say, well actually, the whole theory of evolution based on Darwinism is wrong, and that it’s waiting to be replaced by a different theory of evolution based on some ideas from development. Brian Goodwin quite clearly said that in his book, How the Leopard Changed its Spots.13 So there are kind of two versions of both camps. I think the particularly negative interaction is the one between the evo devo people who I would regard as extreme, who want to completely overthrow Darwinism, and the population geneticists who become then sort of hyper defensive against that and I think that that particular interaction is just a waste of time.
Katrina: What kinds of studies are required to convince skeptics of the evolutionary role of developmental bias?
Wallace: I think there is a job to be done to convince some population geneticists and others of that ilk that development may be capable of playing a positive role in evolution. However, we do have to be careful not to regard any particular group, or even individual, as being homogeneous in their view, as Lewontin illustrated by going from his rather fierce attack on Whyte’s internal selection to his being a party to the spandrels paper.14 Nevertheless, there do remain some people, perhaps many people, who are still resistant to development playing a significant role, and especially a positive role, in directing evolution. What do we need to do to persuade them? That’s a really important question and it consists of many parts: the choice of system, choice of approach, what you’re actually going to try to show, how you’re going to demonstrate it in the best way to convince people, etc. Work by the Brakefield and Jernvall groups, based on particular choices of systems and approaches, have made a good start here. Also, we need to explore the role of bias in the origin of evolutionary novelties. The ongoing work of various groups on the Chelonian (turtle/tortoise) shell are especially interesting in this respect.

1. Gould SJ. 1989. Evolution 43:516-539. 2. Arthur W. 2001. Evol Dev 3:271-278. 3. Minelli A, Bortoletto S. 1988. Biol J Linn Soc 33:323-343. 4. Alberch P, Gale EA. 1983. J Embryol Exp Morph 76:177-197. 5. Alberch P, Gale EA. 1985. Evolution 39:8-23. 6. Beldade P, Koops K, Brakefield PM. 2002. Nature 416:844-847. 7. Allen CE, Beldade P, Zwaan BJ, Brakefield PM. 2008. BMC Evol Biol 8:94. 8. Kavanagh KD, Evans AR, Jernvall J. 2007. Nature 449:427-32. 9. Arthur W. 2004. Cambridge UP. 10. Arthur W. 1997. Cambridge UP. 11. Whyte LL. 1965. Tavistock Publications. 12. Gould SJ. 2002. Harvard UP. 13. Goodwin B. 1996. Touchstone. 14. Gould SJ, Lewontin RC. 1979. Proc R Soc B 205:581-598.