A TVOL interview with Tobias Uller: Orchestrating the Extended Evolutionary Synthesis project
by David Sloan Wilson & Tobias Uller
23 January 2017

This View of Life (TVOL) is featuring a series of articles on the Extended Evolutionary Synthesis, based on a major grant from the John Templeton Foundation awarded to our team of scientists headed by Kevin Laland (St Andrews University) and Tobias Uller (Lund University). This interview with Tobias begins to introduce the empirical side of the project, including his own contribution. The grant as a whole is organized into four themes: (1) conceptual issues, (2) evolutionary innovation, (3) inclusive inheritance, and (4) evolutionary diversification.
David Sloan Wilson: Welcome, Tobias, to TVOL. Please tell us about yourself and how you got involved in this effort.
Tobias Uller: Hi David, and thanks for having me. My background is in evolutionary ecology. This is a field that studies how organisms interact with each other and with their surroundings, and in particular what the evolutionary causes and consequences of those interactions are. Glorified natural history, if you so wish. Much of my work has been concerned with the adaptive significance of plasticity and maternal effects, but I have also worked on the evolution of life histories and transitions between genetic and environmental sex determination, among other things. Ever since my PhD advisor Mats Olsson introduced me to lizards, they have been closest to my heart, but I also work on other animals, most recently water fleas.
Like many others, I was highly influenced by Mary Jane West-Eberhard’s book on developmental plasticity and evolution1, which came out while I was a second-year graduate student. It opened my eyes to thinking about plasticity as both cause and consequence of evolution. Her book also made it clear that evolutionary biology is incredibly rich in conceptual issues that are unresolved, and this encouraged my already strong interest in the history of science and philosophy. My wonderful colleagues let me nurture these interests throughout my postdoctoral years, including a few formative months as a Fulbright Fellow in Alex Badyaev’s lab, and later as a Royal Society University Research Fellow and Departmental Lecturer, both at the Department of Zoology, University of Oxford.
If you add my curiosity for what people with different backgrounds have to say, you will not be surprised to hear that I often end up in contexts where I am the odd one out. It was one of those times, at a philosophy of biology meeting in Oxford, that Kevin Laland asked me what I thought of the proximate-ultimate distinction. I suppose I said something useful because from there on we started working together, often meeting at John Odling-Smee’s house outside of Oxford.
After John and I took part in a public debate in London (organized by the London Evolutionary Research Network), we felt that the lack of a clear statement of what the features of the so-called ‘Extended Evolutionary Synthesis’ actually were seriously hampered communication. People ended up talking past each other. Kevin, John and I decided we needed to spell out the core features of the EES to make progress, which ended up as a Darwin Review in Proceedings of the Royal Society B2.
We think that was a great start, but conceptual frameworks should be evaluated on the basis of the research that they inspire. Trying to make this framework do useful work was the inspiration for the grant. It is truly fantastic that we now are in a position to make this happen.
DSW: Indeed! Before we get to the grant as a whole, please describe some of your own research projects and how they illustrate the EES. Starting out with concrete examples will help to illustrate some of the themes, which by themselves can be quite abstract.
TU: Sure! My projects revolve around the idea that plasticity – that is, environmental effects on phenotypes – can initiate and direct evolutionary diversification. I am involved in theoretical modeling of the relationship between plasticity and evolution in a project headed by Richard Watson at Southampton. But the large majority of my work is empirical, so I’ll give you a couple of examples to illustrate what we are up to.
One of my projects, with evolutionary developmental biologist Nathalie Feiner, will test if plasticity shaped diversification of Anolis lizards. These lizards are textbook examples of an adaptive radiation because, across the Caribbean, a single species gave rise to multiple species, each locally adapted to a different habitat. We are particularly interested in limb morphology since it is a defining feature of adaptive differences between species; lizards that run around on broad surfaces, such as tree trunks, have longer limbs than those who cling onto twigs, for example.
We already know from work by Jonathan Losos and others that limb growth is plastic in Anolis. What we do not know is if evolutionary diversification of limbs took place through modification of those particular components of bones that respond to mechanical stress during growth – as would be predicted if plasticity ‘took the lead’ in evolution – or if adaptive divergence between species is unrelated to plastic responses within species. To test the concordance between plasticity and evolutionary diversity we rear a lot of lizards from several species on different surfaces and combine this with detailed measures of skeletons of very many species across the entire Anolis group.
We should also remember that plastic responses in some cases can carry over to the next generation. In experiments on water fleas, which have the advantage that they can reproduce clonally so we can rear genetically identical individuals in the lab, we will test the hypothesis that such maternal effects (or non-genetic inheritance) facilitate adaptation to new environments. In some ways, this works just like plasticity within a generation. That is, successful accommodation of environmental stressors enables populations to persist and gives natural selection something useful to work with, thereby providing directionality to evolution.
But here there is another twist that has to do with the evolution of inheritance. As populations adapt, selective removal of costs and negative side-effects should make maternal effects behave like signals, sent from mothers to tell offspring about the environment they are likely to encounter. This process, therefore, describes the evolution of a type of inheritance system.
We cannot study the conversion of an environmentally induced stress response to a detection-based inheritance system in the lab. But we can compare water flea populations that have been exposed to the same stressor, such as metals or toxins, for a different number of generations in the wild. Ultimately, this should give insights into how inheritance systems evolve and how they come to transmit information.
DSW: This is very interesting and helpful for understanding the EES in general terms. Now let’s proceed to how the whole grant is organized in terms of themes.
TU: There are four themes – (1) conceptual issues, (2) evolutionary innovation, (3) inclusive inheritance, and (4) evolutionary diversification. Each theme comprises several projects and has a dedicated theme leader who is responsible for ensuring good progress, to facilitate interaction between projects, and to generally stimulate and encourage interesting new leads. The theme leaders are Tim Lewens at Cambridge, Armin Moczek at Indiana, myself at Lund, and Kevin Laland at St Andrews.
DSW: Let’s describe each theme in order.
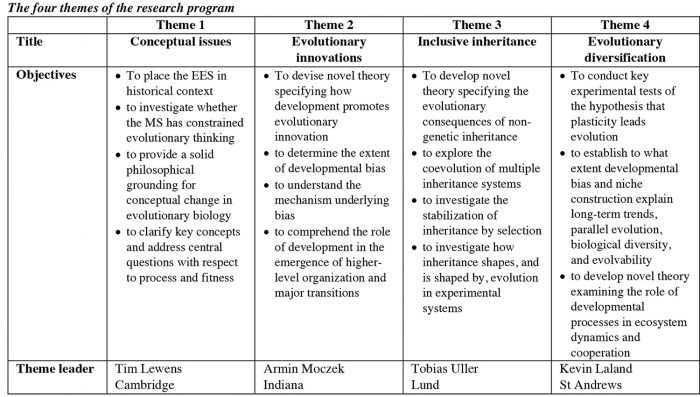
TU: The first theme – on conceptual issues – will place the current discussions about the extended evolutionary synthesis in a historical and philosophical context. The center for this work is Cambridge, and the theme projects are headed by philosopher of science Tim Lewens.
Tracing the historical roots of developmental and ecologically oriented thinking in evolution is really important. It helps us to understand why the structure of evolutionary biology is the way it is and how and why it has changed over time. Only by knowing this can we evaluate the extent to which conceptual frameworks bias what questions we ask and the type of answers we seek.
DSW: I couldn’t agree more. Scientists must understand and cite the history of their subjects in the same way that they must understand and cite the current literature. Anything less will undermine the goals of scientific inquiry.
TU: The EES no doubt possesses deep historical roots, but it nonetheless represents a break with the evolutionary biology tradition in several ways. For example, in our first attempt to represent the conceptual structure of the EES (i.e., the aforementioned Darwin Review), we argued that it is characterized by two features: resisting the temptation to ascribe genes a special causal role in development, and describing the process leading up to adaptation in terms of reciprocal causation between organism and environment.
Much work remains to be done before we know if this is a fruitful and even coherent way to describe evolution. We also need to think about what an alternative structure of evolutionary theory means for central concepts in evolutionary theory, such as adaptation and inheritance. The work in this theme will tackle these issues.
DSW: It’s a great strength of the grant that you have included philosophers and historians of science. I know from my decades-long collaboration with Elliott Sober how much a philosopher can add to the ongoing scientific inquiry.
TU: Absolutely, your work is a good example of how communication between scientists and philosophers of science really can make a difference. We think this is a key point, so the philosophical projects will be pursued in close association with other themes, in particular projects that make use of mathematical modeling. We hope that this will inspire others to take a closer look at the EES and what the current developments in evolutionary biology can teach us about scientific change more generally.
DSW: Right! Human cultural evolution is part of the EES and scientific inquiry is a product of cultural evolution. The more we think of science this way, the better we can make it work. Let’s proceed to theme 2.
TU: The second theme is evolutionary innovation. An early response to Darwin’s emphasis on natural selection was that it does not explain where the adaptive variants come from. We also need a theory of the arrival of the fittest, so to say. This problem has been marginalized for a long time, but people like Andreas Wagner have shown that we have the tools to tackle it3.
We believe that the EES provides a productive conceptual framework for exploring innovation. One of the reasons is that development is seen as constructed rather than programmed. This makes it easier to understand how, for example, environmentally induced variation can play a role in adaptive evolution without the need for the inheritance of acquired characters.
For example, Richard Watson at Southampton is a computer scientist who makes use of an approach known as connectionist learning theory to model evolution4,5. He uses his models to derive predictions about the factors that influence an evolving lineage’s capacity to generate new functional variation.
On the empirical side, we have projects addressing developmental bias6 as a widespread phenomenon, including case studies of horned beetles as well as comparative analyses across taxa. An exciting project at my own institution is Charlie Cornwallis and Per Lundberg’s project on how developmental mechanisms contribute to transitions to multicellularity, one of the major transitions in evolution.
This is also a good example of how we have attempted to build bridges between theory and empirical projects: Andy Gardner (St Andrews) will model such evolutionary transitions in individuality and Graeme Ruxton (also St Andrews) will address similar problems, by asking how exploratory behavior and plasticity shape evolutionary diversification in house building in eusocial insects, another major transition in evolution.
DSW: I look forward to featuring some of these examples in more detail in future TVOL articles in this series. This brings us to the theme 3: an extended concept of inheritance.
TU: Yes. Contemporary evolutionary biology has inherited the Modern Synthesis’ notion of inheritance as transmission of genes. This implies that only genetic mutations can make the transition from being a cause of development to being a cause of evolution.
But this is not the only way to think of heredity. Not all factors that are passed from parents to offspring are copied. A broader notion of heredity does not assume that inheritance can be reduced to gene transmission, but instead goes back to an older notion that heredity arises from similarity in developmental process. Under this perspective, inheritance evolves as development evolves.
DSW: Whoa! Some unpacking is required here! Darwin and his contemporaries knew little more about developmental processes than they did about genes. They defined heredity purely at the phenotypic level, as a resemblance between parents and offspring. You inadvertently used the word in this purely phenotypic sense when you wrote “Contemporary evolutionary biology has inherited the Modern Synthesis’ notion…”. The Modern Synthesis led to a very narrow conception of inheritance centered on genes. Not only did this conception marginalize the role of developmental systems in genetic evolution, but it also marginalized the role of epigenetic processes, forms of social learning found in many species, and forms of symbolic thought that are distinctively human, as summarized by Eva Jablonka and Marion Lamb in their excellent book Evolution in Four Dimensions. All of this is part of the EES, but in what sense is the statement “heredity arises from similarity in developmental process” an older notion?
TU: This is because the view of inheritance as transmission is surprisingly recent in biology. Historians of science have traced the concept of heredity in detail and this shows that development and inheritance were not seen as two distinct autonomous processes until the late 19th century7. The pattern of heredity, that like begets like, was instead explained by reference to similarity of circumstances surrounding conception, pregnancy, birth and so on. It is only with the establishment of genetics as a discipline that the separation of development and inheritance is completed.
It is no doubt useful to treat inheritance as if it was autonomous from development, not the least when it comes to modeling evolution. But it has the unfortunate consequence that inheritance becomes a static channel, whereas in fact systems of inheritance can arise and evolve.
For example, there is evidence that DNA methylation can be heritable across generations. Sometimes this makes new, perhaps stress-induced, variation persist across generations, regardless of whether this is good or bad. In other cases, DNA methylation appears to be an environmentally flexible modification that enable adaptive transfer of information across generations. And sometimes methylation is copied with a fidelity that approaches that of the DNA sequence, making it act as replicator or a ‘gene’ in the pre-molecular understanding of the word (still widely applicable in evolutionary biology) 8.
A question that fascinates me is how we are to understand how the same biological entity or process come to take on these multiple ‘roles’ in development and evolution9,10. This is the motivation for our water flea project. On the theoretical side, Marcus Feldman (Stanford) and Rufus Johnstone (Cambridge) will, in different ways, model how non-genetic inheritance evolves.
Another important aspect of inheritance is the transfer of the micro-organisms we carry in our guts, on our skin and so on. As Scott Gilbert says, we were never individuals! But it is unclear how such individuals-as-communities (sometimes called ‘holobionts’) arise and evolve. Research projects by Armin Moczek and Mike Wade (both Indiana) will attempt to clarify this theoretically and provide empirical insights from horned beetles.
DSW: I’m especially interested in this topic because it interfaces with my earlier work with Bill Swenson on ecosystem selection. We created microbial ecosystems in the laboratory and selected them on the basis of ecosystem-level phenotypic traits, such as the pH of the medium or the ability to break down a toxic chemical11,12. Even though the ecosystems were colonized by millions of organisms from a single well-mixed source, so that initial variation based on sampling error was negligible, they became very different from each other over the course of a single ecosystem ‘generation’ (which comprised many microbial generations). This is because each ecosystem was a highly complex system that embarked on a separate trajectory through phenotypic space, in the same way as the lines of Richard Lenski’s famous long-term experiment on E. coli (read more here). So we had lots of variation. Selection consisted of using the ecosystems at each tail of the phenotypic distribution to inoculate the next generation of ecosystems. The phenotypic distribution of the offspring generation shifted in the direction of selection, which is proof of heritability. In complex systems terms, we were selecting ecosystems based on two properties: 1) the phenotypic properties that were being selected, and 2) enough local stability so that at least some ‘offspring’ ecosystems fall into the same basin of attraction as ‘parent’ ecosystems. No one knew what to make of these experiments when they were published, but now they describe what’s taking place with the microbiomes of every multicellular organism!
TU: There are many interesting parallels between our microbiomes and other ecological communities. But I do not think we understand very well how tightly integrated ecological communities that are self-sustaining evolve. This is another area where the connectionist models from computer science can be put to use, which is one of the projects in our fourth and final theme: the causes of diversification.
DSW: Ok, then let’s proceed with the fourth theme.
TU: From a genetic perspective, it may seem natural to assume that variation is unbiased with respect to function. And if inheritance is merely the passing on of genes, natural selection does all the creative work in evolution.
The EES’s emphasis on development as a cause of adaptive variation means we cannot assume such lack of bias. For example, are some organisms particularly prone to radiate into locally adapted forms because they respond plastically to adaptively relevant features of their environment? Several projects will test predictions from this ‘phenotype-first’ perspective, championed by Mary Jane West-Eberhard, using iconic examples of adaptive diversification, including Susan Foster’s (Clark) project on sticklebacks, Paul Brakefield’s (Cambridge) project on butterflies, and our own work on lizards.
Behaviors are often the first response to environmental change. One project by Erik Svensson (Lund) will focus on how behavioral plasticity shapes the evolution of morphology and coloration by combining field studies, experiments, and comparative analyses of damselflies and beetles.
But developmental bias is not just about character variation. That organisms continuously respond to their environment also means that selection becomes intertwined with development. When organisms affect selective pressures, they participate in evolutionary niche construction and this imposes another source of directionality on evolution. How this occurs will be both modeled and tested empirically.
For example, Kevin Laland and colleagues at St Andrews (Maria Dornelas, David Paterson) will test to what extent niche construction contributes to adaptive trends and parallel evolution using comparative analyses of birds’ nests and spiders’ webs, and experiments in corals and bacteria. It is also interesting to ask if such signals are detectable as macro-evolutionary trends, a question Doug Erwin (Santa Fe) will address by combining mathematical modeling with analyses of the fossil record.
DSW: A veritable feast of projects that I look forward to sharing with TVOL readers in subsequent articles! How will you manage to orchestrate it?
TU: Good question – this is a real challenge, but one we look forward to facing. Kevin and I have drawn from our experience with similarly sized consortia to make sure the coordination of projects will be as helpful as possible in this regard.
For example, rather than bring together people with the hope that they will start to communicate, projects are built on new collaborations, and the close ties between projects make communication both natural and important. These range from complementary theoretical and empirical projects to the close connection between biologists and philosophers of science that run through the program.
We have also included a number of activities that promote the broader picture, including three fully funded workshops. These target different core questions and facilitate interaction with researchers that are not funded by the grant but who will be invited to participate. This includes people who are skeptical to what we are trying to achieve, whose opinion and inputs will be really important to sharpen thinking about the structure of evolutionary theory.
DSW: We will also feature EES skeptics in our series of articles to showcase science as a process of constructive disagreement—in stark contrast to worldwide political processes, I regret to say. Realistically, what do you think that the grant can achieve? After all, £5.7m is a lot of money, and you have clearly pulled together an impressive team, but three years is not a great deal of time.
TU: Too true. And added to that, much of the research is new and innovative, or deploying methods that may not work as envisaged. Realistically, some of the 22 projects are likely to fail and others will not deliver until after the grant period. But what we hope is that a good fraction of the studies succeed in illustrating the merits of these ways of thinking about the relationship between development and evolution, either by generating exciting findings or devising novel methodologies, or by confirming EES predictions. One or other of these projects may even turn out to become a landmark study that changes how researchers think about the evolutionary process.
DSW: Perhaps, but I don’t think that a landmark study will be required to validate the EES. The cumulative progress of all the studies might well be sufficient. Thanks for this overview and I look forward to covering your progress on TVOL.
TU: Thanks, David, we really appreciate your interest.
1. West-Eberhard MJ. 2003. Oxford UP. 2. Laland KN, et al. 2015 Proc R Soc B 282: 20151019. 3. Wagner A. 2011. Oxford UP. 4. Watson RA, et al. 2016. Evol Biol. 43:53-581. 5. Watson RA, Szathmáry E. 2016. Trends Ecol Evol. 31:147-157. 6. Brakefield P. 2006. Trends Ecol. Evol.21:362-368. 7. Müller-Wille S, Rheinberger, HJ. 2012. U Chicago P. 8. Griffiths P, Stotz K. 2013. Cambridge UP. 9. Uller T. 2012. Oxford UP. 10. Uller T, Helanterä H. 2016. Oxford UP. 11. Swenson W, et al. 2000. Environmental Microbiology. 2:564–571. 12. Swenson W, et al. 2000. PNAS. 97:9110-9114.